The author is a consultant to Merck & Co., Inc., GlaxoSmithKline, and Roche Molecular Diagnostics.
New data enhance our knowledge in two critical areas previously covered in this update: the human papillomavirus (HPV) vaccine and HPV DNA testing for cervical cancer screening.
Among findings published in 2007:
- results of phase-3 trials of the quadrivalent and bivalent HPV vaccines, which confirm the remarkable efficacy seen in phase-2 trials among women not previously exposed to the vaccine-targeted HPV types
- three large randomized cervical cancer screening trials from Canada, Sweden, and the Netherlands, which confirm the superiority of cervical cancer screening programs that add HPV DNA testing to cytology in women 30 years and older
- the much-anticipated update of consensus guidelines on the management of women with abnormal cervical cancer screening tests.
Efficacy of HPV vaccine approaches 100% in targeted population
Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927.
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943.
These trials, referred to as FUTURE I and II, were multicenter, multicountry, double-blinded, placebo-controlled trials of the quadrivalent vaccine (Gardasil) that enrolled women 15 to 26 years of age (TABLE).1 Participants were followed for an average of 3 years after receiving the first of the series of three vaccinations. The efficacy of the vaccine at preventing high-grade neoplasia associated with vaccine-targeted HPV types (HPV 6, 11, 16, and 18) was 98% and 100% in the two trials among the “per-protocol,” susceptible population. That population was defined as women who had no evidence of exposure to the targeted HPV types, according to serologic or HPV DNA testing during the first 7 months of the trials, and who received all three vaccinations. This population is a good indicator of how the vaccine will work in adolescents who are not yet sexually active (TABLE).
Efficacy is high in key phase-3 trials of the HPV vaccine
| AUTHORS | HPV TYPES TARGETED | WOMEN (N) | FOLLOW-UP | ENDPOINT | VACCINE EFFICACY (%)* |
|---|---|---|---|---|---|
| Garland et al (FUTURE I) (2007) | 6, 11, 16, 18 | 4,499 | 3 years | CIN 2+ and adenocarcinoma in situ | 100 (95% CI, 94–100) |
| FUTURE II Study Group (2007) | 6, 11, 16, 18 | 12,167 | 3 years | CIN 2+ and adenocarcinoma in situ | 98 (95% CI, 86–100) |
| Joura et al1 (2007) | 6, 11, 16, 18 | 15,596 | 3 years | Vulvar intraepithelial neoplasia 2+ and vaginal intraepithelial neoplasia 2+ | 100 (95% CI, 72–100) |
| Paavonen et al (2007) | 16, 18 | 15,626 | 15 months | CIN 2+ | 90 (95% CI, 53–99) |
| * In women naïve to vaccine-targeted HPV types by serology and HPV DNA testing. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia. | |||||
The vaccine was less effective in the “intention-to-treat” population that included all women enrolled in the study regardless of HPV status. At 3 years, the vaccine reduced high-grade neoplasia associated with vaccine-targeted HPV types in this population by only 29% and 50% in the two trials.
When these results were published, some experts expressed concern and questioned the benefit of the vaccines.2 There is no reason for concern, however, because lower short-term efficacy in the intention-to-treat population was expected. Because the current generation of HPV vaccines does not have a measurable therapeutic effect, vaccination will not prevent cervical intraepithelial neoplasia (CIN) in women who are already infected with vaccine-targeted HPV types; nor will it cause regression of CIN lesions that are already present when the woman is vaccinated.
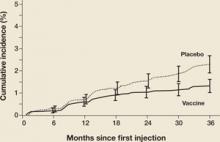
There were a number of women already infected with vaccine-targeted HPV types at enrollment in the “intention-to-treat” population. Some of these women developed CIN 2,3 associated with vaccine-targeted HPV strains during the first 18 months of the trial (FIGURE 1). However, with longer follow-up, the cumulative number of cases of CIN 2,3 plateaued in vaccinated women, whereas it continued to rise in the placebo arm. Thus, with longer follow-up, vaccine efficacy will improve. Therefore, both the American College of Obstetricians and Gynecologists and the Advisory Committee on Immunization Practices recommend that all sexually active adolescents and young women be vaccinated through 26 years of age.
Figure 1 Cases of CIN 2,3 eventually plateau in vaccinated women
The graph charts the efficacy of the quadrivalent HPV vaccine in preventing CIN 2,3 in the intention-to-treat population of a phase-3 efficacy trial.
SOURCE: Garland et al. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Bivalent vaccine was 90.4% effective against CIN 2,3