What’s the most common cause of death in women 40 to 44 years of age?
Answer: Breast cancer.1
What population has the highest death toll from breast cancer?
Answer: Women age 40 and older. Approximately 95% of new breast cancer cases and 97% of breast cancer deaths occur in women 40 and older.2
Why are many women in their 40s under-screened?
Answer: Because clinicians sometimes under-appreciate their risk.
Of course, the majority of breast cancer cases and deaths involve postmenopausal women. But this doesn’t mean younger women don’t warrant heightened scrutiny. This article presents 4 cases that focus on breast disease in women 40 to 49 years of age and the optimal workup for patients with suspicious findings. It includes recommendations on:
Digital mammography may aid in evaluation of dense breast tissue
Although early breast cancer may be more difficult to identify on mammography in premenopausal women because of their denser breast tissue, there are good data on the benefits of screening mammography for women in the 40- to 49-year-old age group,3,4 as well as for women age 50 and older.5 In addition, digital mammography is often helpful in women with radiographically dense breasts.
Screening vs diagnostic mammography
Women who have no complaints and no abnormal physical findings on self- or clinical examination typically undergo screening mammography. In typical cases, 2 views of each breast are obtained, and the radiologist often postpones reading the images until the end of the day, when they are scrutinized in batches.
In contrast, diagnostic mammography is performed when a possible problem arises, and several additional “coned-down” views may be needed. The radiologist interprets the study while the patient is still in the radiology office.
It is essential that gynecologists indicate on the mammography referral form whether they are requesting a diagnostic or screening mammogram. If it is a diagnostic mammogram, the reason and precise location of any suspicious areas need to be clearly communicated to the radiologist. In either case, the patient should be reminded to provide any previous images the radiologist does not already have.
I obtain routine annual screening mammography for my average-risk patients from age 40 onward, since more than 50,000 American women under age 50 are diagnosed with breast cancer each year.
CASE 1 Abnormal mammogram
S.H. is a healthy 43-year-old who had vaginal deliveries at ages 25 and 28 and has always used barrier contraception. She has no family history of cancer or high-risk factors for breast cancer. Her breast and pelvic examinations at the time of her routine gynecologic visit are normal. She has undergone annual mammography since she was 40 years old, but recently moved from another state and did not bring her mammograms with her.
She is sent for a screening mammogram, and 2 radiographic views of each breast are obtained. Upon review, the images are classified as Breast Imaging Reporting and Data Systems (BI-RADS) category 0, indicating that mammographic assessment is incomplete. The radiologist wants additional images of an area in the upper outer quadrant of the right breast, and wants to compare this study with the patient’s old films if they are available. S.H. returns the next day for the additional imaging, and the radiologist identifies an 8-mm area of suspicious calcifications in the right breast, reclassifying the mammogram as BI-RADS category 4.
The radiologist recommends a stereotactic biopsy, but S.H. wants your advice on whether to comply or proceed immediately to open biopsy.
What do you tell her?
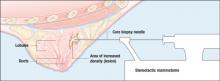
Barely invasive biopsy
Stereotactic needle biopsy offers precise positioning in 3 dimensions without the lag time and scarring associated with open biopsies.In the case above, stereotactic biopsy is a good option, since it can usually be scheduled rapidly, does not require an operating room or anesthesia, is less expensive than open biopsy, and involves less scarring.6 If a patient’s lesion is clearly benign on stereotactic biopsy, she may be spared an open biopsy. If it is malignant, she can immediately begin to make treatment decisions.
Breast imaging categories
BI-RADS categories have standardized the reporting of mammograms and include the following7:
S.H. schedules a stereotactic biopsy for the following day in the radiologist’s office. Tissue diagnosis reveals an invasive ductal carcinoma, and the patient elects to undergo lumpectomy and radiation therapy.