- Luteal phase deficiency (LPD), defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids.
- Wide variation in the reported incidence of LPD—3.7% to 20% in infertile women—reflects lack of agreement about its diagnostic criteria.
- Histologic dating of an endometrial sample is the gold standard for evaluation of the corpus luteum.
- Two main treatment strategies have been suggested: improving follicular dynamics using follicle-maturing drugs such as clomiphene, and use of supplemental progesterone during the luteal phase and first trimester of pregnancy.
Despite scanty and controversial supporting evidence, evaluation of patients with infertility or recurrent pregnancy loss for possible luteal phase deficiency (LPD) is firmly established in clinical practice. In this article, we examine the data and offer our perspective on the role of LPD in assessing and managing couples with reproductive disorders (FIGURE 1).
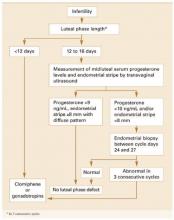
FIGURE 1 Diagnosis and treatment of luteal phase deficiency
Many areas of controversy
Although observational and retrospective studies have reported a higher incidence of LPD in women with infertility and recurrent pregnancy losses than in fertile controls,1-4 no prospective study has confirmed these findings. Furthermore, studies have failed to confirm the superiority of any particular therapy.
Once considered an important cause of infertility, LPD has become the subject of debate, with some experts questioning its very existence. Unclear terminology describing this disorder is part of the problem, making it difficult to definitively diagnose the deficiency or determine its incidence. Further, while reasonable consensus exists that endometrial biopsy is the most reliable diagnostic tool, concerns remain about its timing, repetition, and interpretation.
A defect of corpus luteum progesterone output?
LPD is defined as endometrial histology inconsistent with the chronological date of the menstrual cycle, based on the woman’s next menses. It was first described by Jones in 1949.5 One year later, Noyes et al6 published criteria on endometrial dating that became the gold standard for LPD diagnosis.
Pathophysiology. LPD may be caused by deficient progesterone secretion from the corpus luteum or failure of the endometrium to respond appropriately to ovarian steroids (TABLE). Most experts believe LPD is a defect of corpus luteum progesterone output—both in amount and duration—resulting in inadequate stimulation of the endometrium for implantation of the blastocyst (FIGURE 2).5 Thus, the endometrial histologic pattern is an important bioassay of the corpus luteum steroidogenic function.
Normal embryonic implantation depends on a properly functioning luteal phase, which, in turn, requires optimal secretion of follicle-stimulating hormone (FSH) and adequate follicular development during the follicular phase. Other requirements are a satisfactory luteinizing hormone (LH) surge during ovulation and continuous tonic LH pulses during the luteal phase of the cycle.
LH secretion from the pituitary occurs in a pulsatile fashion,7 which is essential for corpus luteum function.8 During the follicular phase the pulse frequency is high, occurring at a rate of approximately 1 pulse per 90 minutes. However, during the luteal phase and under the influence of progesterone, the pulse frequency is significantly diminished, occurring approximately every 3 to 6 hours, depending on the age of the corpus luteum.7,9 The corpus luteum is unresponsive to LH pulses during the early luteal phase; sensitivity develops about 4 to 6 days after ovulation.7,9
The luteal phase thus involves creation of an optimal hormonal environment as well as adequate endometrial transformation. Alteration of any of the factors that contribute to a normally functioning corpus luteum may thus deleteriously affect the endometrium and embryonic implantation.
Epidemiology. The reported prevalence of LPD ranges from 3.7% to 20% among patients with infertility.10,11 When an out-of-phase endometrium is the diagnostic criterion, prevalence estimates range from 3.5% to 31%.12,13 A short luteal phase is thought to occur in 5% of ovulatory cycles.14Some reports suggest that LPD accounts for 25% to 40% of recurrent pregnancy losses.15
The wide variation in reported incidence of LPD reflects the lack of agreement about its definition and diagnostic criteria. Further, some studies evaluating prevalence have not concurrently tested controls—an important omission since endometrial histology suggestive of LPD occurs in up to 50% of single menstrual cycles and 25% of sequential cycles.16 Thus, the true incidence of the defect may never be known.
TABLE
Etiology of luteal phase deficiency
| FOLLICULAR PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic ovarian defects |
|
| Intrinsic endometrial defects |
|
| LUTEAL PHASE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects |
|
| Intrinsic secretory endometrial defect |
|
| LUTEAL RESCUE EVENTS |
| Trophic alterations |
|
| Intrinsic corpus luteum defects in early pregnancy |
|
| Intrinsic endometrial defects |
| FSH = follicle-stimulating hormone, hCG = human chorionic gonadotropin, |
| LH = luteinizing hormone |